Release date:2020-07-31
Allergy
[IF:6.771]
Real-world evidence of subcutaneous allergoid immunotherapy in house dust mite-induced allergic rhinitis and asthmaDOI: 10.1111/all.14240
Abstract:
Background: The objective of this study was to analyze the effectiveness of allergen immunotherapy (AIT) with an allergoid in the treatment of house dust mites (HDM)-induced allergic rhinitis and/or asthma based on recent real-life data. The outcomes were measured using asthma incidence and consumption of corresponding medications as the indicator of persisting symptoms.
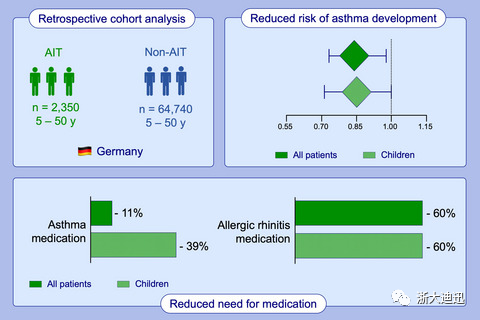
Methods: In this retrospective cohort analysis of a German longitudinal prescription database, patients who received at least two relevant mite AIT prescriptions in two different successive seasonal cycles were compared with non-AIT patients who received at least three symptomatic allergic rhinitis (AR) prescriptions in successive mite seasons. Study endpoints included AR progression, asthma progression, asthma occurrence, and therapy adherence. We used multivariate regression analyses to estimate the effects of AIT, adjusting for relevant variables.
Results: This study included 2350 patients receiving a mite allergoid and 64 740 control patients. After up to 6 years of follow-up, patients treated with mite allergoid required significantly fewer AR and asthma prescriptions (59.7% vs 10.8%) than the control group, and the probability of asthma development was significantly lower. The adherence of patients receiving allergoid was 63.8% at the end of the second year and 38.6% at the end of the third year.
Conclusion: This real-world evidence confirms the good efficacy of subcutaneous AIT with HDM mite allergoid in the treatment of allergic rhinitis and/or asthma. Up to 6 years of follow-up revealed significant effects in allergic rhinitis by measuring the number of AR medications and demonstrating significant reductions in asthma medications.
First Author:
Marek Jute
Correspondence:
Marek Jutel, All-MED Medical Research Institute, Wrocław, Poland.
All Authors:
Marek Jutel, Bernd Brüggenjürgen, Hartmut Richter, Christian Vogelberg
2020-07-31 Article
 hth官方网页版中国有限公司
hth官方网页版中国有限公司
