Release date:2019-02-20
JACI:Volume 143, Issue 1, January 2019, Pages 66-73
[IF:13.1]
Controversies in drug allergy: Testing for delayed reactionshttps://doi.org/10.1016/j.jaci.2018.10.030
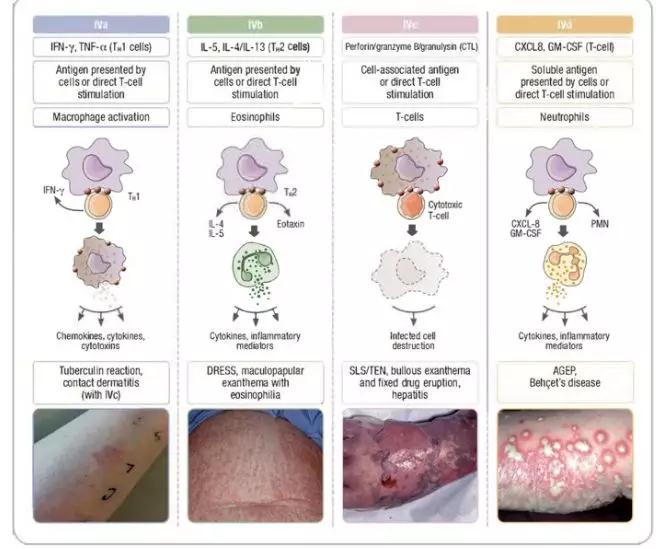
Controversies exist with regard to in vivo approaches to delayed immunologically mediated adverse drug reactions, such as exanthem (maculopapular eruption), drug reaction with eosinophilia and systemic symptoms, acute generalized exanthematous pustulosis, Stevens-Johnson syndrome/toxic epidermal necrolysis, and fixed drug eruptions. In particular, widespread differences exist between regions and practice on the availability and use of intradermal and patch testing, the standard drug concentrations used, the use of additional drugs in intradermal and patch testing to help determine cross-reactivity, the timing of testing in relation to the occurrence of the adverse drug reaction, the use of testing in specific phenotypes, and the use of oral challenge in conjunction with delayed intradermal and patch testing to ascertain drug tolerance. It was noted that there have been advances in the science of delayed T cell–mediated reactions that have shed light on immunopathogenesis and provided a mechanism of preprescription screening in the case of HLA-B*57:01 and abacavir hypersensitivity and HLA-B*15:02 and carbamazepine Stevens-Johnson syndrome/toxic epidermal necrolysis in Southeast Asian subjects. Future directions should include the collaboration of large international networks to develop and standardize in vivo diagnostic approaches, such as skin testing and patch testing, combined with ex vivo and in vitro laboratory approaches.
All Author:
ElizabethJ.PhillipsMDab∗PaulBigliardiMDcAndreasJ.BircherMDdAnaBroylesMDeYoon-SeokChangMD,PhDfWen-HungChungMD,PhDgRannakoeLehloenyaMBChBhMajaMockenhauptMD,PhDiJonnyPeterMBChB,Mmed,PhDhMunirPirmohamedFRCP,PhDjJean-ClaudeRoujeauMDkNeilH.ShearMDlLucianaKaseTannoMD,PhDmnJasonTrubianoMBBS,PhDopRoccoValluzziMDqAnnickBarbaudMD, PhDrs∗
2019-1-17 review
 hth官方网页版中国有限公司
hth官方网页版中国有限公司
