Release date:2020-11-26
Allergy
[IF:6.771]
Quality of life of children aged 8-12 years undergoing food allergy oral immunotherapy: Child and parent perspectiveDOI: 10.1111/all.14350
Abstract:
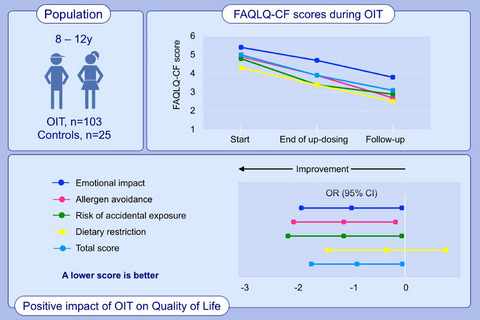
Background: Oral immunotherapy (OIT) for food allergy improves the quality of life (QOL) of children from parental perspective but little is known about the child perception.
Methods: The Food Allergy Quality of Life Questionnaire—Child Form (FAQLQ-CF) was administered to children aged 8-12 years, and the FAQLQ—Parent Form (FAQLQ-PF) was administered to their parents at the start of OIT for milk, egg, peanut, sesame, or tree nuts, at the end of up-dosing, and after 6 months of follow-up. Food-allergic children not undergoing OIT served as controls. Children QOL scores were compared to their parents.
Results: The total FAQLQ-CF score of 103 children undergoing OIT improved significantly from start of OIT (median (IQR); 4.8, 3.8-5.7) to end of up-dosing (3.9, 3-5.2) (P < .001). A greater improvement was noted in the 56 children who reached a followup visit, from 5.0 (3.7-5.8) at OIT start to 3.1 (1.8-5.0) on follow-up, (P < .001). In contrast, FAQLQ-CF scores of control patients improved mildly and nonsignificantly between the two time points from 5.3 (4.3-5.7) to 4.8 (3.6-6.0), (P = .13). The improvement in the total FAQLQ-CF scores from OIT start to follow-up was significantly greater compared to the change in control patients during observation (P = .015).
Parents reported better QOL scores compared to their children at all stages of OIT (start 4.0, 3.2-5, P = .004; end of up-dosing 2.9, 1.9-4.7, P = .04; follow-up 2.2, 1.6-3.6, P = .003).
Conclusion: QOL of food-allergic children undergoing OIT improves significantly compared to controls. Parents perceive QOL to be better than the perception of the children.
First Author:
Na'ama Epstein-Rigbi
Correspondence:
Na’ama Epstein-Rigbi, The Institute of Allergy, Asthma and Immunology, Shamir Medical Center (former Assaf Harofeh), Zerifin 71300, Israel.
All Authors:
Na'ama Epstein-Rigbi, Michael R. Goldberg, Michael B. Levy, Liat Nachshon, Arnon Elizur
2020-11-26 Article
 hth官方网页版中国有限公司
hth官方网页版中国有限公司
